Resources
- Resources and References for Managed Care and Payer Professionals
- A Payer’s Guide to the Hemophilia Comprehensive Care Model
- Hemophilia Outcomes Tool Box
- National Hemophilia Foundation (NHF)
- Hemophilia Treatment Centers (HTCs)
- Centers for Disease Control and Prevention (CDC)
- 340B Drug Program and Hemophilia
- Forms and Templates
- Patient Resources
- Patient Support Groups
Resources and References for Managed Care and Payer Professionals
Elucidating the Value of HTC-delivered Care in the Management of Bleeding Disorders addresses topics such as:
- total cost of hemophilia
- value of HTC-delivered care for payers
- effectiveness of HTC-delivered comprehensive care
- role and benefit of 340B discount drug pricing
This resource can help managed care and payer professionals when presenting the benefits of the comprehensive care model within their organization or with various stakeholders.
A Payer’s Guide to the Hemophilia Comprehensive Care Model
Click here to download the Payer’s Guide to the Hemophilia Comprehensive Care Model
The Payer’s Guide to Comprehensive Hemophilia Management contains valuable information regarding care models and treatment approaches in hemophilia, as well as the potential health plan implications of these interventions. This resource can help managed care stakeholders better understand the prevailing clinical management strategies in hemophilia and serves as a reference that can be used to educate colleagues.
Hemophilia Outcomes Tool Box
The Hemophilia Outcomes Tool Box contains tools and best practice resources to measure outcomes in hemophilia. This resource can help managed care organizations evaluate success in managing hemophilia and provides a reference tool that can be used to educate colleagues.
National Hemophilia Foundation (NHF)
NHF is an organization dedicated to finding better treatments and cures for inheritable bleeding disorders and to preventing the complications of these disorders through education, advocacy and research. Established in 1948, the NHF has chapters throughout the United States (US). Its programs and initiatives are made possible through the generosity of individuals, corporations and foundations as well as through a cooperative agreement with the Centers for Disease Control and Prevention (CDC).
Hemaware – The Bleeding Disorders Magazine
Hemaware – The Bleeding Disorders Magazine is sponsored by NHF and is published quarterly. It covers topics on: life stages of hemophilia, research and treatment, health and wellness, parenting and family, women and hemophilia, and also has a community pulse section featuring blogs, videos, and photos. To subscribe, go to: www.hemaware.org/subscribe
Advocacy
www.hemophilia.org/Advocacy-Healthcare-Coverage
NHF advocates for the needs and interests of people affected by hemophilia and related bleeding and clotting disorders. As a result of efforts at the federal level, millions of dollars of government funds have been devoted to improve medical care, services, education, and safety and surveillance of blood and blood products. Other efforts have raised awareness of bleeding disorders, protected individuals from discrimination and ensured full access to high-quality care. Advocacy at NHF goes beyond Washington and Capitol Hill by working closely with chapters and associations, and other key stakeholder groups across the country to address community needs at the state and local levels. To learn more about the NHF’s various advocacy initiatives, go to the following websites.
- Advocacy priorities:
www.hemophilia.org/Advocacy-Healthcare-Coverage/Advocacy-Priorities - Advocacy resources:
www.hemophilia.org/Advocacy-Healthcare-Coverage/Advocacy-Tools-Resources - Take Action:
www.hemophilia.org/Advocacy-Healthcare-Coverage/Advocacy-Tools-Resources/6-Steps-For-Grassroots-Advocacy - Health Care Reform:
www.hemophilia.org/Advocacy-Healthcare-Coverage/Advocacy-Priorities/State-Priorities/Healthcare-Reform-Implementation
Medical and Scientific Advisory Council
www.hemophilia.org/Researchers-Healthcare-Providers/Medical-and-Scientific-Advisory-Council-MASAC
NHF has a medical advisory council to advance clinical care and promote hemophilia research.
Known as the Medical and Scientific Advisory Council (MASAC), this body establishes quality of care guidelines for the treatment of hemophilia and other bleeding disorders. Issued in the form of recommendations, MASAC guidelines set the standard of care around the world and are referred to by an international array of physicians, medical schools, pharmacists, emergency room personnel, insurance companies and others. The scientists, physicians and other treatment professionals who comprise MASAC are internationally regarded as experts in the broad field of bleeding disorders research and care, AIDS, hepatitis, other infectious diseases and blood safety. MASAC meets twice a year to issue its recommendations, which are then approved by NHF’s Board of Directors. MASAC recommendations are posted on NHF’s Web site, are available to callers through NHF’s information clearinghouse (800-42-HANDI or handi@hemophilia.org), and are published in NHF’s HemAware magazine.
MASAC Guidelines Recommendation #188 for Pharmacy Providers
MASAC has recommendations regarding standards of service for pharmacy providers of clotting factor concentrates for home use to patients with bleeding disorders.
The guidelines recommend that any pharmacy provider dispensing clotting factor concentrates for home use provide services that meet the minimal standards.
- Pharmacy Provider Staff Knowledge of Clotting Factor
- Concentrates and Ancillary Supplies
- Clotting Factor Concentrates and Ancillaries
- Processing of Prescription Orders
- Hours of Operation/Access to Staff
- Delivery
- Recordkeeping, Billing and Product Recall
For a list of the 35 specialty pharmacies self-reporting having met or exceeded MASAC Recommendation #188 Standards, go to:
www.hemophilia.org/NHFWeb/MainPgs /MainNHF.aspx?menuid=57&contentid=1107
Additional Key MASAC Recommendations Include the Following:
- Guidelines for Emergency Department Management of Individuals with Hemophilia (Document #175)
www.hemophilia.org/NHFWeb/MainPgs /MainNHF.aspx?menuid=57&contentid=691
- Recommendations Concerning Products Licensed for the Treatment of Hemophilia and Other Bleeding Disorders (Document #215)
www.hemophilia.org/NHFWeb/MainPgs /MainNHF.aspx?menuid=57&contentid=693
- Standards and Criteria for the Care of Persons with Congenital Bleeding Disorders (Document #132)
www.hemophilia.org/NHFWeb/MainPgs /MainNHF.aspx?menuid=57&contentid=220
- Recommendations Regarding Factor Concentrate Prescriptions and Formulary Development and Restrictions (Document #159)
www.hemophilia.org/NHFWeb/MainPgs /MainNHF.aspx?menuid=57&contentid=179
- Recommendation Concerning Prophylaxis (Regular Administration of Clotting Factor Concentrate to Prevent Bleeding (Document #179)
www.hemophilia.org/NHFWeb/MainPgs /MainNHF.aspx?menuid=57&contentid=1007
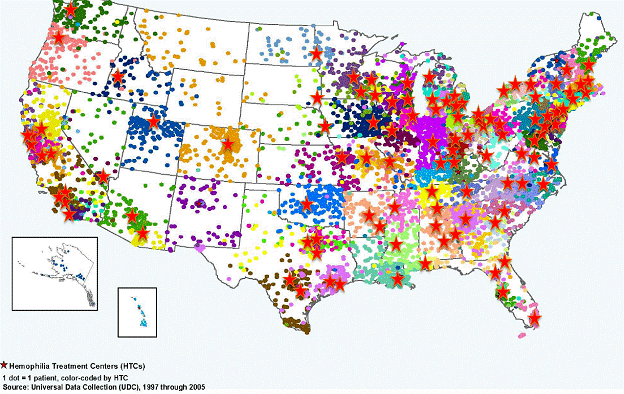
Hemophilia Treatment Centers (HTCs)
https://www.cdc.gov/ncbddd/hemophilia/htc.html
Many people with bleeding disorders use the resources of a hemophilia treatment center (HTC) because the staff understands their unique needs. The hematologists, nurses, psychosocial professionals and physical therapists not only help consumers with their medical care issues, but also lend tremendous emotional support. Individuals who go to hemophilia treatment centers will find state-of-the art medical care, and also an experienced staff that takes time to develop comprehensive treatment care plans for patients and families. HTCs are located in cities across the U.S.
HTCs are spread through the U.S. in areas with high patient concentration.
Members of the care team at HTCs include:
- Hematologists—specialists in blood disorders.
- Pediatricians—specialists in caring for infants, young children, and teenagers.
- Nurses—medical specialists in hemophilia care. The nurse is probably the person you will see most frequently.
- Social Workers—specialists who assist you with the issues of daily living, such as adjusting to hemophilia and locating resources (e.g., insurance, transportation, housing, etc.).
- Physical therapists—specialists in activity, exercise, and rehabilitation.
- Orthopedists—specialists in disorders of the bones and joints.
- Dentists—specialists in disorders of the teeth and gums. The dentists at HTCs are experts in treating children with oral bleeding problems.
- You are also an important member of the treatment team. The staff needs your input to develop a plan of care that will ensure your remain healthy, active, and able to live successfully with added challenge of hemophilia.
To search for a HTC, regional HTC coordinators, or staff members from specific HTCs, go to the Centers for Disease Control and Prevention (CDC) link: https://www.cdc.gov/ncbddd/hemophilia/htc.html and search through the fields provided. You can also request a printed copy of the U.S. Hemophilia Treatment Centers Directory, at 800-42-HANDI or handi@hemophilia.org.
Centers for Disease Control and Prevention (CDC)
www.cdc.gov/ncbddd/ hemophilia/index.html
The CDC Hemophilia homepage contains various materials and resources for patients, parents, care providers, and health systems. Hemophilia affects 1 in 5,000 male births. About 400 babies are born with hemophilia each year. The exact number of people living with hemophilia in the United States is not known. A CDC study conducted in six states in 1994 estimated that about 17,000 people had hemophilia at that time. Currently, the number of people with hemophilia in the United States is estimated to be about 20,000, based on expected births and deaths since 1994.
To access the CDC’s many useful initiatives, go to the following websites.
- Facts: www.cdc.gov/ncbddd/ hemophilia/facts.html
- Treatment: www.cdc.gov/ncbddd/ hemophilia/treatment.html
- Data & Statistics: www.cdc.gov/ncbddd/ hemophilia/data.html
- Training & Education: www.cdc.gov/ncbddd/ hemophilia/training.html
- Research: www.cdc.gov/ncbddd/ hemophilia/research.html
- Universal Data Collection: www.cdc.gov/ncbddd/ blooddisorders/udc/index.html
- Blood Safety: www.cdc.gov/ncbddd/ hemophilia/bloodsafety.html
- Inhibitors: www.cdc.gov/ncbddd/ hemophilia/inhibitors.html
- Recent Articles: www.cdc.gov/ncbddd/ hemophilia/articles.html
- Free Materials: www.cdc.gov/ncbddd/ hemophilia/freematerials.html
- Videos: www.cdc.gov/ncbddd/ hemophilia/video.html
Real Stories from People living with Hemophilia:
www.cdc.gov/ncbddd/ hemophilia/stories.html
- Links to Other Websites: www.cdc.gov/ncbddd/ hemophilia/links.html
Universal Data Collection System (UDC)
One of the major challenges of rare disorders, such as hemophilia, is lack of uniform health data. To address this issue, CDC created a national public health surveillance project called the Universal Data Collection (UDC) system. UDC is carried out with the help of federally funded HTCs in the U.S. and its territories. To access UDC Data Reports available to the public, go to: https://www.cdc.gov/ncbddd/hemophilia/htc.htmlwww2a.cdc.gov/ncbddd/ htcweb/UDC_Report/UDC_Report.asp
340B Drug Program and Hemophilia
The 340B Drug Pricing Program requires drug manufacturers to provide outpatient drugs to eligible health care organizations/covered entities at significantly reduced prices. To participate in the 340B Program, eligible organizations/covered entities are registered and enrolled with the 340B program and comply with all 340B Program requirements. Covered entities are assigned a 340B identification number that vendors verify before allowing an organization to purchase 340B discounted drugs.
340B pharmacy providers are an important component of comprehensive hemophilia care. Many patients with hemophilia can get their factor and other medically necessary supplies from a 340B pharmacy provider. These entities, including many HTCs, vary in size and offer a range of services. Health plans or their pharmacy benefit manager will contract with a 340B pharmacy provider to deliver factor and supplies, hemophilia education materials and programs, as well as emergency telephone support.
For a list of eligible organizations/covered entities participating in the 340B Drug Pricing Program, go to: www.hrsa.gov/opa/ eligibilityandregistration/index.html
Forms and Templates
Treatment Log Example
- Captures relevant patient-reported data
- Monitors infusions
- Tracks bleed activity
- Outlines trends and target joint issues
- Provides HTC with relevant clinical information
| Dosage Information | Comments | Bleed Locations | Reason for Treatment (circle one) |
Did you miss any school or work as a result of this bleed? If yes, how many days? | ||
| Key locations (circle one) | Other locations (circle one or specify in writing) | |||||
| Date: Time: Name of factor: Lot#: Dose (total number of units infused): |
Head Stomach |
Mouth Thigh GI Upper arm Lower arm Other: |
Prophylaxis Surgery Physical therapy Injury Preventative infusion (Infused before playing sports, etc.) Spontaneous |
Yes No How many days of school? How many days of work? |
||
| LEFT | RIGHT | |||||
| Hand Elbow Shoulder Hip Knee Ankle Foot |
Hand Elbow Shoulder Hip Knee Ankle Foot |
|||||
| Date: Time: Name of factor: Lot#: Dose (total number of units infused): |
Head Stomach |
Mouth Thigh GI Upper arm Lower arm Other: |
Prophylaxis Surgery Physical therapy Injury Preventative infusion (Infused before playing sports, etc.) Spontaneous |
Yes No How many days of school? How many days of work? |
||
| LEFT | RIGHT | |||||
| Hand Elbow Shoulder Hip Knee Ankle Foot |
Hand Elbow Shoulder Hip Knee Ankle Foot |
|||||
Patient Resources
Program Assistance Availability
https://www.patientservicesinc.org/ For-Patients/Supported-Illnesses
Hemophilia Education
http://www.kelleycom.com/
Patient Support Groups
National Bleeding Disorders Foundation (NBDF)
NHF is an organization dedicated to finding better treatments and cures for inheritable bleeding disorders and to preventing the complications of these disorders through education, advocacy and research.
Hemophilia Federation of America (HFA)
The HFA is a consumer advocate for safe, affordable, and obtainable blood products and health coverage, as well as a better quality of life for all persons with bleeding disorders. HFA’s ongoing consumer advocacy agenda includes product safety, as well as accessibility, affordability, and availability of the products the individuals of this community require.